Fujifilm Healthcare Americas Corporation recently announced its innovative colonic polyp detection technology, CAD EYE, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This artificial intelligence (AI) powered system enhances the detection process of potential pre-cancerous lesions, regardless of size, shape and color within the colon during standard colonoscopies. Here’s everything you need to know about this groundbreaking technology.
Key Highlights
- Real-time detection: CAD EYE identifies subtle colonic mucosal lesions like polyps and adenomas during colonoscopies.
- Improved accuracy: Increases detection rates of these potentially pre-cancerous lesions compared to traditional methods.
- Efficiency: Assists endoscopists without increasing procedure time.
- Expert level analysis: CAD EYE’s detection accuracy is comparable to expert endoscopists.
What is CAD EYE?
CAD EYE is an AI-powered software designed to work with Fujifilm’s endoscopic imaging systems. During a colonoscopy, the software analyzes the endoscopic images in real time. If it detects a suspicious mucosal lesion, CAD EYE alerts the endoscopist with a visual indicator (Detection Box) and a sound signal. This helps minimize the possibility of missed lesions, leading to more effective early detection and prevention of colorectal cancer.
Consisting of a compatible expansion unit (the Fujifilm EX-1) and endoscopy support software (EW10-EC02), CAD EYE is an evolution of Fujifilm’s ELUXEO Endoscopic Imaging System, featuring AI image processing functionality customized for the integration with the system’s processor and the endoscope.
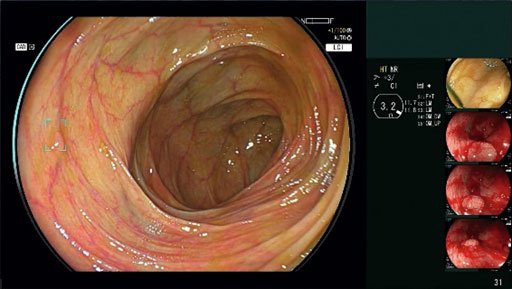
Why is This Important?
Colorectal cancer is a leading cause of cancer death, but it’s highly preventable with early detection and removal of pre-cancerous polyps. CAD EYE aims to significantly improve the accuracy of colonoscopies, making them an even more powerful tool in cancer prevention. Studies indicate the technology helps detect more adenomas during screening and surveillance procedures.
Expert Opinions
Dr. Sravanthi Parasa, a gastroenterologist at Swedish Medical Center, expressed enthusiasm about the FDA approval: “This breakthrough not only enables the early detection of precancerous lesions that can lead up to colorectal cancer but also significantly reduces the risk of missed lesions, enhancing our precision and improving patient outcomes.”
The Future of Colon Cancer Screening
Fujifilm’s CAD EYE technology represents a powerful new tool in preventative medicine. This advancement could make colon cancer screening more effective and accessible, ultimately saving lives.