The U.S. Food and Drug Administration (FDA) recently granted 510(k) clearance to Apple Watch’s innovative AFib History feature, marking a significant step in utilizing consumer wearables in medical research and patient monitoring. This regulatory approval highlights the potential of the Apple Watch to serve not just as a fitness tracker but as a crucial tool in managing and studying cardiac health.
Breakthrough in Cardiac Health Monitoring
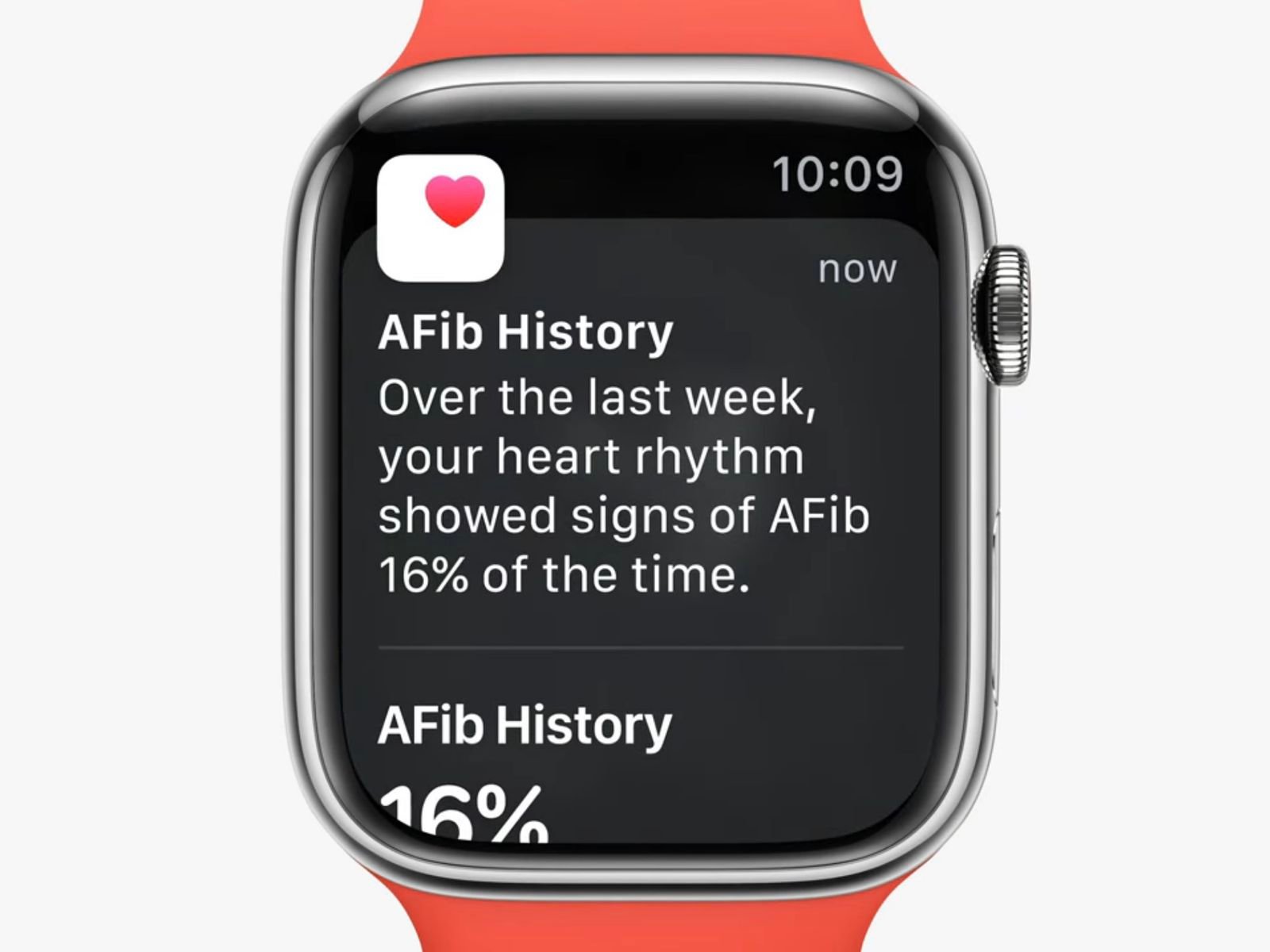
The newly approved AFib History feature on the Apple Watch allows individuals diagnosed with atrial fibrillation (AFib) to track the frequency of their heart irregularities over time. This feature is part of Apple’s broader strategy to enhance its device’s health monitoring capabilities, making it a valuable asset for both users and healthcare providers.
FDA’s Stamp of Approval
The FDA’s approval specifies that the AFib History feature can be activated on Apple Watches running watchOS 9 or later, by users aged 22 and older who have been previously diagnosed with AFib. The clearance was part of a larger update that also included other health monitoring technologies such as sleep tracking and medication management tools.
How It Works
To set up the AFib History tracking, users need to navigate through the Health app on their paired iPhone, confirming their AFib diagnosis as part of the process. Once enabled, the feature provides weekly summaries of AFib frequency, which can be reviewed directly from the Health app. These insights are crucial for understanding how lifestyle factors such as sleep, exercise, and alcohol consumption might influence one’s cardiac rhythm.
Data Privacy and Usage
Apple emphasizes the ease of sharing this health data with physicians, aiming to foster better-informed medical consultations. Users can export their AFib history as a PDF, making it straightforward to share with healthcare professionals.
Implications for Clinical Studies
With this FDA clearance, the AFib History feature is not only a tool for personal health monitoring but also qualifies for use in clinical studies. This development opens new doors for research into atrial fibrillation and could potentially accelerate the adoption of wearable technology in clinical settings.